Posts: 5,270
Threads: 99
Joined: Aug 2013
So I tried the coconut and cuke water, yuk! lol. Then I figured ok, chia seeds (omega 3) check, and still yuk!. Oh.....why not take a green tea extract capsule and add it in?, check.....oh wait!!.....Cinnamon!, (antioxidant) ok, alright I can do this.

Posts: 5,270
Threads: 99
Joined: Aug 2013
The foam rises at first, but after adding the rest of the ingredients it blended quite well.
Posts: 5,270
Threads: 99
Joined: Aug 2013
The foam rises at first, but after adding the rest of the ingredients it blended quite well.
Posts: 5,270
Threads: 99
Joined: Aug 2013
Repost:
(13-11-2014, 11:29 PM)Lotus Wrote: (13-11-2014, 09:38 PM)iaboy Wrote: (13-11-2014, 06:35 PM)Lotus Wrote: So, what if we knew when (the time of day) E was released and the amount, would it change you're NBE strategy?.
I would think to some degree. If your Andros are way high, you would then use stronger or more AA. Thereby maybe even lowering your progesterone and estrogens?? I would think, if the last statement would be true, then it would be safer, quicker and more economical????
Good points, I haven't seen anything yet to suggest when it's more beneficial, attack or defend cycles. I think we have to look at the "lock and key theory" to understand what can work better. When there's an excess (let's say it's E, choose the flavor, nbe or hrt) the binding process (which causes growth) can't all activate at the same time, so the excess just flops around and does nothing, it's a waste. The question is for how long does it stay that way (yes, that would be half-life's) the goal is to examine that process so we can affect a more efficient and economical cycle, (meaning a daily one).
![[Image: attachment.php?aid=8342]](http://www.breastnexus.com/attachment.php?aid=8342)
And here's our transports-
Posts: 5,270
Threads: 99
Joined: Aug 2013
Repost:
(13-11-2014, 11:29 PM)Lotus Wrote: (13-11-2014, 09:38 PM)iaboy Wrote: (13-11-2014, 06:35 PM)Lotus Wrote: So, what if we knew when (the time of day) E was released and the amount, would it change you're NBE strategy?.
I would think to some degree. If your Andros are way high, you would then use stronger or more AA. Thereby maybe even lowering your progesterone and estrogens?? I would think, if the last statement would be true, then it would be safer, quicker and more economical????
Good points, I haven't seen anything yet to suggest when it's more beneficial, attack or defend cycles. I think we have to look at the "lock and key theory" to understand what can work better. When there's an excess (let's say it's E, choose the flavor, nbe or hrt) the binding process (which causes growth) can't all activate at the same time, so the excess just flops around and does nothing, it's a waste. The question is for how long does it stay that way (yes, that would be half-life's) the goal is to examine that process so we can affect a more efficient and economical cycle, (meaning a daily one).
![[Image: attachment.php?aid=8342]](http://www.breastnexus.com/attachment.php?aid=8342)
And here's our transports-
Posts: 5,270
Threads: 99
Joined: Aug 2013
1 more repost as it relates to the last one.
(15-12-2014, 09:58 PM)Lotus Wrote: The following is some info already posted but should mentioned again for its importance, (highlighted text in particular)
(19-11-2014, 02:54 AM)Lotus Wrote: It is now known that estrogens exert their end-organ effect by activating a complex intracellular mechanism. Tissues which respond to estrogen possess intracytoplasmic proteins (receptors) that preferentially bind specific steroids.
For instance, a cell from the uterus will possess 5000–15,000 estrogen receptors whereas a cell from the spleen will have none. These receptors recognize estrogens by their three dimensional and chemical characteristics and bind it with high affinity (KD =10-10), specificity, and saturability.
The estrogen molecules present in the circulation are relatively loosely bound to intravascular carrier proteins (sex-steroid-binding globulin [SBG]) (KD = 10-8) or to albumin. In excess of 95% of the estrogen in the circulation is found in the bound form. The estrogen readily diffuses across the cell membrane in its active free form due to a concentration and a binding gradient. The estrogen molecule is relatively small (molecular weight is 300) and lipophilic and probably passes through the cell membrane by simple diffusion.
Once in the cell, the estrogen is promptly bound to the intracellular (intracytoplasmic) receptor protein, which then undergoes a series of complex spatial changes prior to intranuclear transport. This nuclear transport occurs within 30–45 minutes after the target tissue is exposed to estrogen. The following system of nuclear interactions between receptor and DNA is a model that has been proposed by McCarty.3 The activated receptor–estrogen complex then nonspecifically binds to the DNA and protein of dispersed chromosomes (euchromatin) and stimulates acetylation of the histone protein.
This acetylation of the histones in nucleosomes causes the nucleosome to “open up” and expose specific DNA segments for transcription. The “estrogen message” is transcribed into new messenger RNA which then migrates back into the cytoplasm and activates various cellular processes including new protein synthesis. The now “freed” receptor protein is probably recycled back into the cytoplasm for further use.
The estrogen receptor recognizes a molecule as being “estrogen” if its size, three-dimensional configuration, and charge are similar to the parent molecule. Therefore, the nonsteroidal synthetic estrogens may not resemble the “prototype” estrogen (estradiol-17β) on paper diagrams but are very similar in shape and other properties as seen by the cellular receptor. Estrogen receptors are perhaps the determinants of potency for estrogenic substances.
The estrogen receptors preferentially bind estradiol over estriol (2x) and estrone (3x).5 This receptor also discriminates among the estrogens by binding estradiol within the cellular nucleus longer than the weaker estrogens estriol and estrone.
Therefore, estradiol is the most potent of the natural estrogens probably because of the greater affinity and duration of its receptor-binding compared with the other available estrogens. Receptors for estrogen and other steroid hormones can now be accurately quantified and studied. Estrogen in physiologic concentrations stimulates the synthesis of estrogen receptors and of progesterone and testosterone receptors.
Progesterone and testosterone, however, inhibit estrogen receptors. Progesterone inhibits its own receptor population in the secretory phase of human endometrium. Thus, it is apparent that for estrogen to bind and influence a tissue, the specific estrogen receptors must be present. The potency of a particular estrogen in a tissue roughly parallels and is probably dependent on the quantity of the estrogen receptor in the cells of that tissue. Studies with estrogen receptors in breast cancers are being successfully utilized to predict the responsiveness of these tumors to hormonal manipulation.
http://www.glowm.com/section_view/item/2.../value/237
-------------------------------
The adrenal gland is the primary source of estrogen in postmenopausal women, and estrone is the dominant estrogen, the E2:E1 ratio being reversed after menopause. In comparison to those of cycling women, estrone levels are reduced to low follicular phase levels. There is an insignificant contribution to the estrone pool from estradiol conversion, ovarian estrone secretion, and conversion of ovarian androstenedione
However, virtually all the total estrone production can be accounted for by peripheral conversion of androstenedione in adipose tissue and liver. There is a strong correlation with age and obesity in the conversion efficiency of androstenedione to El. The nonobese postmenopausal woman has an average androstenedione to E1 conversion rate of 2.7%, compared with 5.1% for the obese postmenopausal patient with uterine bleeding secondary to increased endogenous estrogen.
Posts: 5,270
Threads: 99
Joined: Aug 2013
1 more repost as it relates to the last one.
(15-12-2014, 09:58 PM)Lotus Wrote: The following is some info already posted but should mentioned again for its importance, (highlighted text in particular)
(19-11-2014, 02:54 AM)Lotus Wrote: It is now known that estrogens exert their end-organ effect by activating a complex intracellular mechanism. Tissues which respond to estrogen possess intracytoplasmic proteins (receptors) that preferentially bind specific steroids.
For instance, a cell from the uterus will possess 5000–15,000 estrogen receptors whereas a cell from the spleen will have none. These receptors recognize estrogens by their three dimensional and chemical characteristics and bind it with high affinity (KD =10-10), specificity, and saturability.
The estrogen molecules present in the circulation are relatively loosely bound to intravascular carrier proteins (sex-steroid-binding globulin [SBG]) (KD = 10-8) or to albumin. In excess of 95% of the estrogen in the circulation is found in the bound form. The estrogen readily diffuses across the cell membrane in its active free form due to a concentration and a binding gradient. The estrogen molecule is relatively small (molecular weight is 300) and lipophilic and probably passes through the cell membrane by simple diffusion.
Once in the cell, the estrogen is promptly bound to the intracellular (intracytoplasmic) receptor protein, which then undergoes a series of complex spatial changes prior to intranuclear transport. This nuclear transport occurs within 30–45 minutes after the target tissue is exposed to estrogen. The following system of nuclear interactions between receptor and DNA is a model that has been proposed by McCarty.3 The activated receptor–estrogen complex then nonspecifically binds to the DNA and protein of dispersed chromosomes (euchromatin) and stimulates acetylation of the histone protein.
This acetylation of the histones in nucleosomes causes the nucleosome to “open up” and expose specific DNA segments for transcription. The “estrogen message” is transcribed into new messenger RNA which then migrates back into the cytoplasm and activates various cellular processes including new protein synthesis. The now “freed” receptor protein is probably recycled back into the cytoplasm for further use.
The estrogen receptor recognizes a molecule as being “estrogen” if its size, three-dimensional configuration, and charge are similar to the parent molecule. Therefore, the nonsteroidal synthetic estrogens may not resemble the “prototype” estrogen (estradiol-17β) on paper diagrams but are very similar in shape and other properties as seen by the cellular receptor. Estrogen receptors are perhaps the determinants of potency for estrogenic substances.
The estrogen receptors preferentially bind estradiol over estriol (2x) and estrone (3x).5 This receptor also discriminates among the estrogens by binding estradiol within the cellular nucleus longer than the weaker estrogens estriol and estrone.
Therefore, estradiol is the most potent of the natural estrogens probably because of the greater affinity and duration of its receptor-binding compared with the other available estrogens. Receptors for estrogen and other steroid hormones can now be accurately quantified and studied. Estrogen in physiologic concentrations stimulates the synthesis of estrogen receptors and of progesterone and testosterone receptors.
Progesterone and testosterone, however, inhibit estrogen receptors. Progesterone inhibits its own receptor population in the secretory phase of human endometrium. Thus, it is apparent that for estrogen to bind and influence a tissue, the specific estrogen receptors must be present. The potency of a particular estrogen in a tissue roughly parallels and is probably dependent on the quantity of the estrogen receptor in the cells of that tissue. Studies with estrogen receptors in breast cancers are being successfully utilized to predict the responsiveness of these tumors to hormonal manipulation.
http://www.glowm.com/section_view/item/2.../value/237
-------------------------------
The adrenal gland is the primary source of estrogen in postmenopausal women, and estrone is the dominant estrogen, the E2:E1 ratio being reversed after menopause. In comparison to those of cycling women, estrone levels are reduced to low follicular phase levels. There is an insignificant contribution to the estrone pool from estradiol conversion, ovarian estrone secretion, and conversion of ovarian androstenedione
However, virtually all the total estrone production can be accounted for by peripheral conversion of androstenedione in adipose tissue and liver. There is a strong correlation with age and obesity in the conversion efficiency of androstenedione to El. The nonobese postmenopausal woman has an average androstenedione to E1 conversion rate of 2.7%, compared with 5.1% for the obese postmenopausal patient with uterine bleeding secondary to increased endogenous estrogen.
Posts: 5,270
Threads: 99
Joined: Aug 2013
Supplements that can Damage the Liver
Experts warn that some herbal and dietary supplements have side effects, especially when taken in large doses or if they are taken for long periods. Research shows that almost 20% of all liver injuries recorded from 2003-2011 are associated with chronic intake of certain herbal and dietary supplements.
http://www.naturalliverdetox.net/dietary...-the-liver
What's not listed is the amounts of pesticides and heavy metals, I've only seen one report on PM and stated the amounts. Although it wasn't toxic amounts, but when you consider how we ramp up our dosages of PM it then should be examined.
Balance, when we experience toxic liver systems it's time to back off, eg. cycle, or detox. Adding in a safe guards like a natural sources of progesterone (like Peggy listed below) its protection you can't afford to miss:
(02-01-2015, 09:14 PM)peggy Wrote: I too want to increase my progesterone level, but naturally. I just found this website and thought I could post it here:
These methods have been shown effective for increasing progesterone levels:
* 750 mg vitamin C per day (increased progesterone 77% and improved fertility)
* 600 mg vitamin E (increased progesterone in 67% of patients)
* 6 g L-arginine (increased progesterone in 71% of patients)
* Increasing beta carotene (boosts progesterone levels in dogs and goats)
* Increasing vitamin B6
* Supplementing with Vitex Agnus Castus (increases progesterone and fertility)
* 120 mg Black Cohosh on days 1 to 12 (increases progesterone and fertility)
* Supplementing with selenium
* Consuming dairy products (shown to raise progesterone in men)
* Weight loss
* Avoiding overeating
* Improving insulin sensitivity (metformin increases progesterone levels 246%)
* Replacing saturated fat in the diet with unsaturated fat
* 80mg progesterone cream (shown to be as effective as 200 mg oral progesterone prescription)
* Eating a high protein, low carbohydrate diet
* Lowering TSH levels in subclinical hypothyroidism
https://sites.google.com/site/miscarriag...ogesterone
I am already taking Vitamin C (1000mg), Selenium and a vitamin B complex since a long time. I dont think these vitamins help to increase my progesterone level. :-/
Vitamin E, L-Arginine and black cohosh sounds interesting though. Ella, if you remember I took black cohosh last year, but only 40mg per day. Maybe I should give it another chance from day 1-12 next cycle. What do you think?
Posts: 5,270
Threads: 99
Joined: Aug 2013
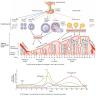
We can see when it makes sense to add in estrogenic and progesterone sources from this chart,.
![[Image: attachment.php?aid=8924]](http://www.breastnexus.com/attachment.php?aid=8924)
Posts: 5,270
Threads: 99
Joined: Aug 2013
Fatty Acid Classifications By Saturation
Fatty acids are classified in the following way:
Saturated: A fatty acid is saturated when all available carbon bonds are occupied by a hydrogen atom. They are highly stable, because all the carbon-atom linkages are filled-or saturated-with hydrogen. This means that they do not normally go rancid, even when heated for cooking purposes. They are straight in form and hence pack together easily, so that they form a solid or semisolid fat at room temperature. Your body makes saturated fatty acids from carbohydrates and they are found in animal fats and tropical oils.
Monounsaturated: Monounsaturated fatty acids have one double bond in the form of two carbon atoms double-bonded to each other and, therefore, lack two hydrogen atoms. Your body makes monounsaturated fatty acids from saturated fatty acids and uses them in a number of ways.
Monounsaturated fats have a kink or bend at the position of the double bond so that they do not pack together as easily as saturated fats and, therefore, tend to be liquid at room temperature. Like saturated fats, they are relatively stable. They do not go rancid easily and hence can be used in cooking. The monounsaturated fatty acid most commonly found in our food is oleic acid, the main component of olive oil as well as the oils from almonds, pecans, cashews, peanuts and avocados.
Polyunsaturated: Polyunsaturated fatty acids have two or more pairs of double bonds and, therefore, lack four or more hydrogen atoms. The two polyunsaturated fatty acids found most frequently in our foods are double unsaturated linoleic acid, with two double bonds-also called omega-6; and triple unsaturated linolenic acid, with three double bonds-also called omega-3. (The omega number indicates the position of the first double bond.)
Your body cannot make these fatty acids and hence they are called "essential." We must obtain our essential fatty acids or EFA's from the foods we eat. The polyunsaturated fatty acids have kinks or turns at the position of the double bond and hence do not pack together easily. They are liquid, even when refrigerated.
The unpaired electrons at the double bonds makes these oils highly reactive.
They go rancid easily, particularly omega-3 linolenic acid, and must be treated with care. Polyunsaturated oils should never be heated or used in cooking. In nature, the polyunsaturated fatty acids are usually found in the cis form, which means that both hydrogen atoms at the double bond are on the same side.
All fats and oils, whether of vegetable or animal origin, are some combination of saturated fatty acids, monounsaturated fatty acids and polyunsaturated linoleic acid and linolenic acid. In general, animal fats such as butter, lard and tallow contain about 40-60% saturated fat and are solid at room temperature.
Vegetable oils from northern climates contain a preponderance of polyunsaturated fatty acids and are liquid at room temperature. But vegetable oils from the tropics are highly saturated. Coconut oil, for example, is 92% saturated. These fats are liquid in the tropics but hard as butter in northern climes. Vegetable oils are more saturated in hot climates because the increased saturation helps maintain stiffness in plant leaves. Olive oil with its preponderance of oleic acid is the product of a temperate climate. It is liquid at warm temperatures but hardens when refrigerated.




